
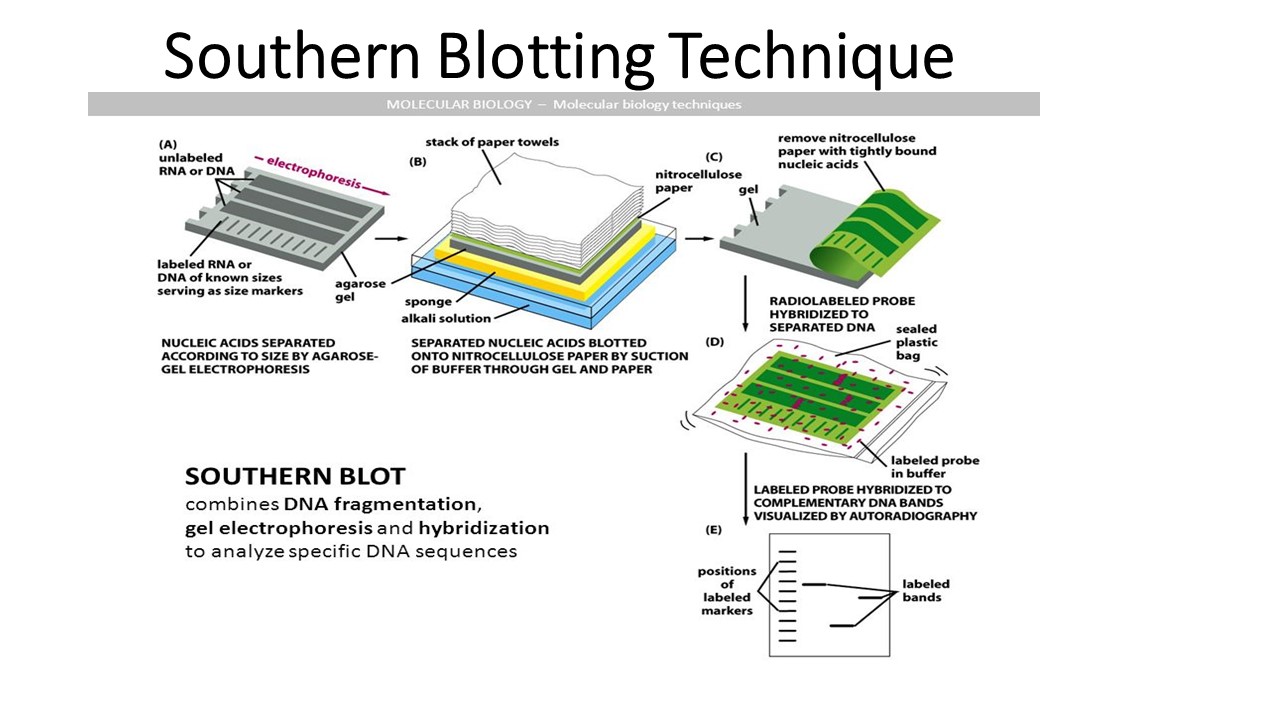
If transferring by suction, 20X SSC buffer is used to ensure a seal and prevent drying of the gel. Pressure is applied evenly to the gel (either using suction, or by placing a stack of paper towels and a weight on top of the membrane and gel), to ensure good and even contact between gel and membrane.
A sheet of nitrocellulose (or, alternatively, nylon) membrane is placed on top of (or below, depending on the direction of the transfer) the gel. The choice of alkaline over neutral transfer methods, however, is often empirical and may result in equivalent results. The denaturation in an alkaline environment may improve binding of the negatively charged thymine residues of DNA to a positively charged amino groups of membrane, separating it into single DNA strands for later hybridization to the probe (see below), and destroys any residual RNA that may still be present in the DNA. If alkaline transfer methods are used, the DNA gel is placed into an alkaline solution (typically containing sodium hydroxide) to denature the double-stranded DNA. This depurinates the DNA fragments, breaking the DNA into smaller pieces, thereby allowing more efficient transfer from the gel to membrane. If some of the DNA fragments are larger than 15 kb, then prior to blotting, the gel may be treated with an acid, such as dilute HCl. The DNA fragments are then electrophoresed on an agarose gel to separate them by size. Restriction endonucleases are used to cut high-molecular-weight DNA strands into smaller fragments.





 0 kommentar(er)
0 kommentar(er)
